First MaxWater paper published in "Angewandte Chemie"
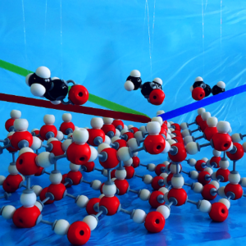
Interactions of trace gases with ice and water surfaces play a major role in atmospheric chemistry. The chemical and photochemical processes of trace gases absorbed on ice surfaces are relevant for ozone depletion and alter the chemical composition of the atmosphere. Specifically, small-oxygenated organic molecules, such as acetone, are a critical contributor to the formation of HOx radicals. Here, we combine surface-specific vibrational spectroscopy and a controllable flow cell apparatus to investigate the molecular adsorption of acetone onto the basal plane of single crystalline Ih ice with large surface area. By comparing the adsorption of acetone on the ice/air with the water/air interface, we find two different types of acetone adsorption, as apparent from the different responses of both the free O-H and the hydrogen-bonded network vibrations for ice and liquid water. Adsorption on ice occurs preferentially through interactions with the free OH group, while the interaction of acetone with the surface of liquid water appears less specific.












